In a previous post (link here), we explained the imminent introduction of a web-based Reading Platform. This new tool is now available to subscribers ! It is designed for users to access the Safety Observer reports in replacement of the pdf reports distributed by email until now.
This page includes 3 short video tutorials to explain how the Platform works.
How to use the Platform
This 6 minute-video provides a tour of the Reading Platform and describes the main features from a User perspective. It presents the interface, the navigation, and how to perform searches.
Access to the Platform
This 5 minute-video explains the steps for users to access the Reading Platform. As mentioned earlier, each user can access the platform from only 2 devices and this video also demonstrates how users can manage the authorized devices in their User Account.
Please Note that the Privacy Settings of your browser may cause problems when trying to access the Reading Platform and we invite you to check this to prevent issues (see Separate Post).
User Management
This 4 minute-video explains the role of “Account Manager”, which only applies to multi-user subscriptions. It demonstrates how to deactivate users and how to add new users to the list of users covered by the subscription for a particular client.
Release Notes:
This section presents the list of improvements implemented since the Platform was launched in December 2023. Please Note that the video tutorials presented above do not show these enhancements, as they were introduced after the videos were produced.
22-Jan-2024: “Account Managers” can now see the status of their Users: From the tab “Users”, a new column “Onboarded” indicates whether the user has activated his/her account. There are also new search fields available to search for specific users in the list.
11-Mar-2024: The following changes were implemented:
- Login page: The field for the email is no longer case sensitive
- Login page: A link “Forgot password ?” is now available for users to reset their password
- User Account: Users can no longer make changes to their email address (only Account Managers and System Administrators can make changes to the email address of other Users)
- User Account: The information now includes the end date of the subscription
- User Account: “Account Managers” can no longer delete a User (only System Administrators can)
- User Account / Browsers: The list of authorized browsers now shows when they were authorized
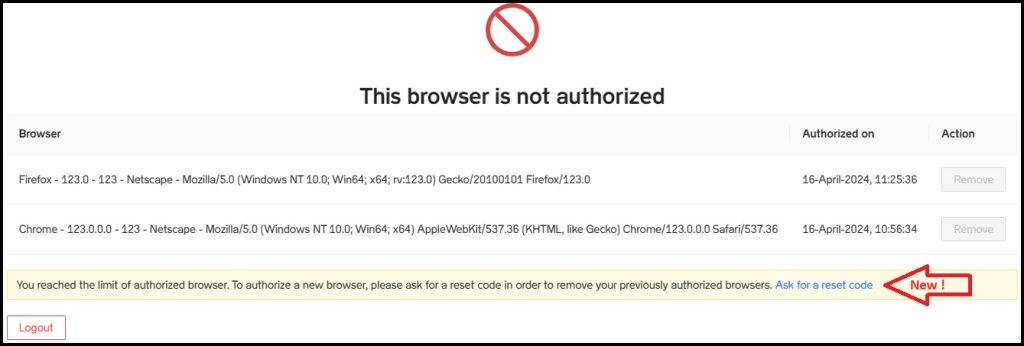
16-Apr-2024: The following change was implemented:
- Browser reset: In the event that users reach the limit of authorized browsers on their account, they now have the possibility to reset it themselves. Instead of contacting support and wait for the authorized browsers to be removed, they can now request a reset code (as shown below). The reset code will be immediately sent to their mailbox. Following reset, the users can again access the Platform after they authorize their browser.